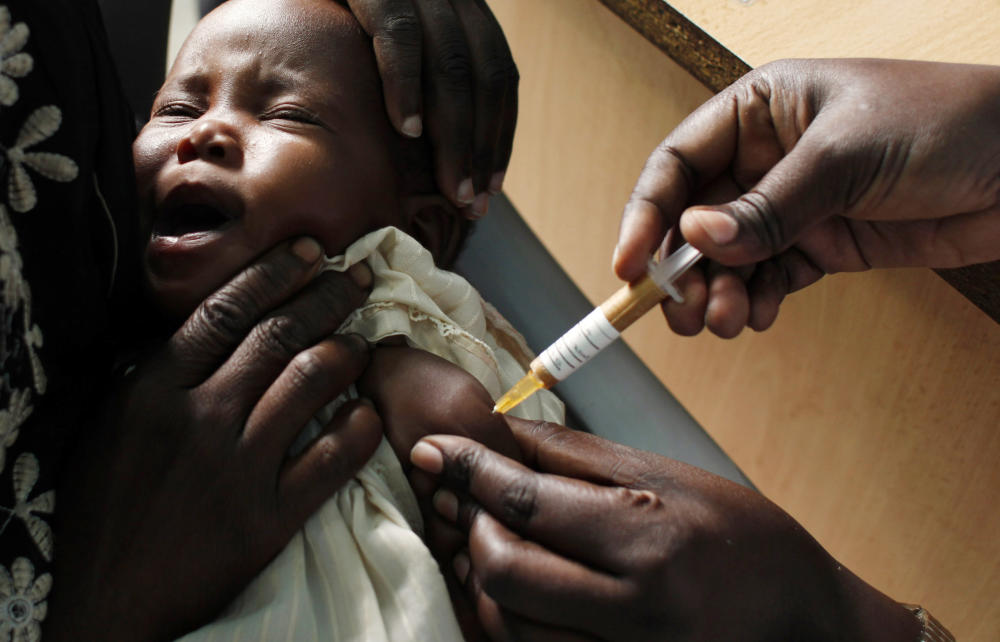
Malaria sickens tens of millions each year and kills roughly 500,000, mainly in Africa. A vaccine has been seen as the holy grail in global efforts against the disease.
Today, the European Medicines Agency recommended for approval a malaria vaccine. It’s been in the works for three decades. It’s called RTS,S — the initials stand for the component parts of the vaccine; it’s also known as Mosquirix. And it’s noteworthy for being the first human vaccine ever against a parasite, targeting Plasmodium falciparum.
The recommendation for approval paves the way for its use in the developing world. Unfortunately, the vaccine turns out to have more than a few limitations. Yet scientists are confident it can still make a huge difference in the fight against malaria.
Unlike vaccines for measles or polio that work more than 90 percent of the time, this new vaccine has an efficacy rate between 26 and 36 percent.
“I think from an objective level most are disappointed that it wasn’t more effective. But this is what we have,” says Nobel laureate Peter Agre, head of the Johns Hopkins Malaria Research Institute.
Then there’s the matter of dosage. In a study of 15,000 infants and young children in Africa, RTS,S only showed a statistically significant benefit against severe malaria in kids who were injected with four doses over the course of 18 months.
The places that need this vaccine the most — the countries with the highest rates of malaria — tend to be the places with the most dysfunctional health systems.
“Currently most countries across sub-Saharan Africa, where the malaria burden is the greatest, do not have a mechanism for delivering three or four doses of a vaccine in the second year of life,” says William Moss, a professor of epidemiology at Johns Hopkins. The timing of those shots is out of synch with the delivery of other vaccines.
One more thing. Even though Glaxo Smith Kline has pledged to sell the vaccine at manufacturing costs plus 5 percent, that’s still an additional financial burden for already impoverished nations.
But despite the vaccine’s drawbacks, Moss believes it is still important.
“I think this could be a game changer in specific settings,” he says.
Stanley Plotkin, a professor emeritus at the University of Pennsylvania and an editor of the medical reference book Vaccines, says getting a parasite vaccine to market is a major step forward in immunology. Plotkin adds that a vaccine could help provide “herd immunity” against malaria. By driving down the number of infections, there will be fewer parasites available to spread and that should mean fewer cases of disease.
“One can hope that the employment of a vaccine even though its primary efficacy may be low will have a public health effect that will be larger than one would expect from the vaccine itself,” he says.
In a study published early this year, the Lancet concluded that RTS,S could make a “substantial” contribution to fighting malaria if used in combination with other tactics that can help control the disease.
So the expectation among researchers is that, even if RTS,S is rolled out on a large scale, the other current measures against malaria — notably bed nets and mosquito control programs — will have to continue to be used if significant progress is going to be made against this tropical disease.
And even if it isn’t the holy grail, the vaccine is making history by spurring immunity against a parasite.
“Parasites are extraordinarily complex organisms,” says Moncef Slaoui, the head of vaccines at Glaxo Smith Kline, has worked on this project ever since he joined the company 27 years ago. “In this instance, this parasite has three forms of life that are totally disconnected.”
The challenge for Slaoui and his colleagues was to come up with a vaccine that blocked the parasite at each stage of its life cycle. The breakthrough for GSK was figuring out how to shut down the parasite inside the human liver.
9(MDA3MTA1NDEyMDEyOTkyNTU3NzQ2ZGYwZg004))